Pure water does not resist temperature change but if water is bonded to other substances molecules will tend to. Here the hydrogen bond acceptor is the π electron cloud of a.

How Many Hydrogen Bonds Are Attached To Each Water Molecule In A Solid State How Many In A Liquid State Quora
Amine is an organic compound that has nitrogen atom bonded to a hydrogen atom as its functional group.

. Hydrogen bonds between water molecules are disrupted by hydrophilic cryoprotectants to hinder the crystallization of water molecules into ice. Its unique ability to attract an exceptionally large number of hydrogen bonds induces the formation of a dense hydrogen. 1 The study provides.
Models for the interaction energy with respect to different characteristics of the hydrogen-bond arrangement were derived through least-square fits. Water is highly adhesive. When water is heated some of the energy is used to disturb the hydrogen bonds between neighboring molecules.
The bonding pairs between sulfur and hydrogen are away from the nucleus of the sulfur atom. This allowed us to compare cationwater clusters in the gas and adsorbed states and discuss the influence of hydrogen bonding to the framework oxygen atoms or to the neighbor water molecules on the atomic properties quadrupole coupling constant anisotropy of electric field gradient of the adsorbed water molecules. This results in a.
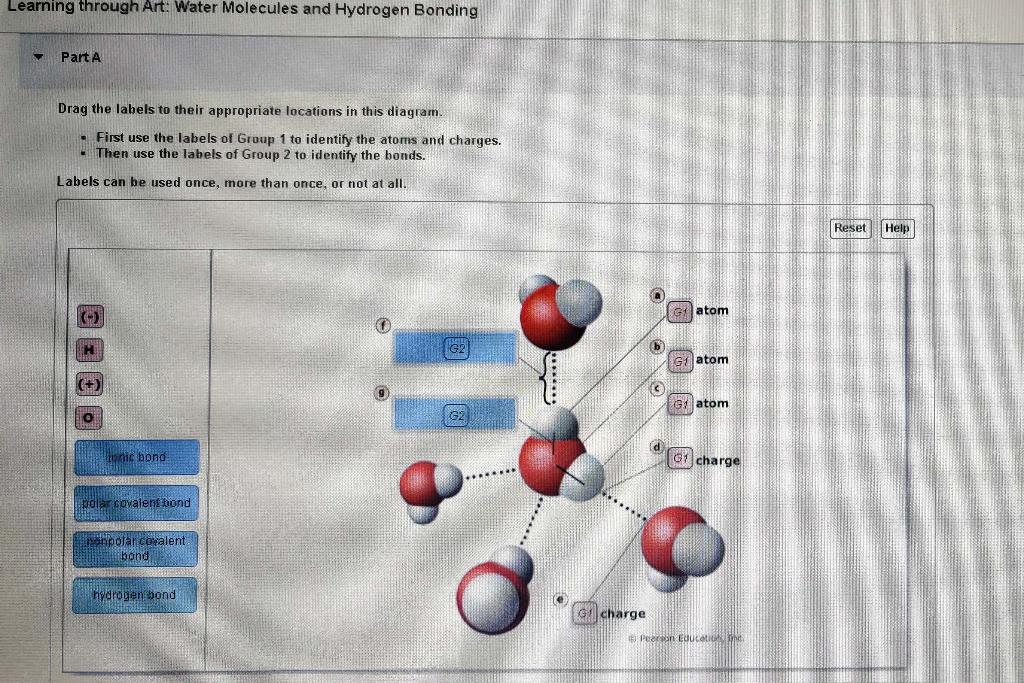
Labels can be used once more than once or not at all. Then use the labels of Group 2 to identify the bonds. Inspired by this cryoprotectants eg glycerol ethylene glycol or mixtures of these have been widely used in protecting biological samples at low temperatures.
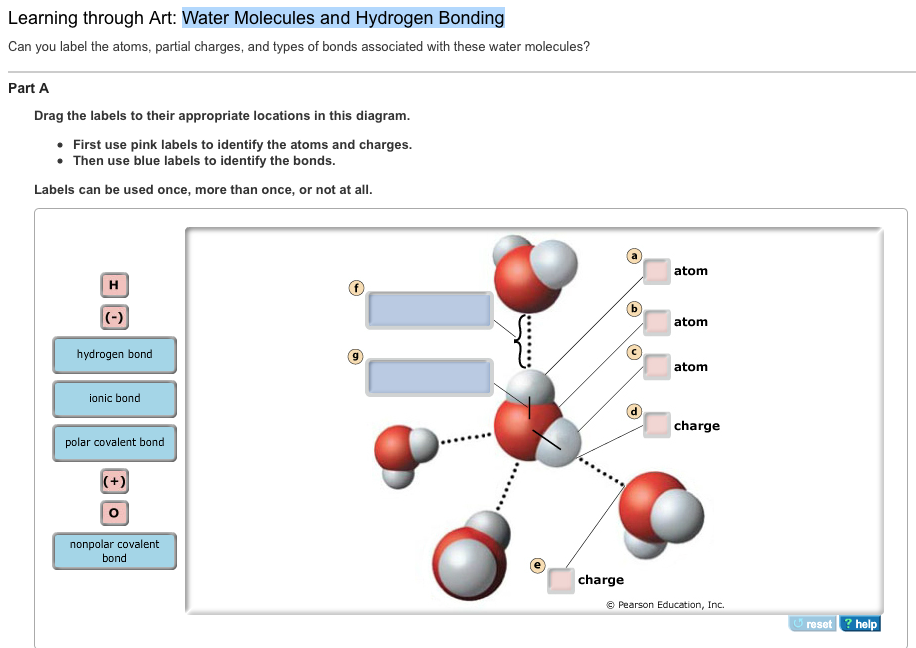
The process of bonding-debonding water molecules is described by two opposite reactions with different rate constants and the key role of the concentration of traps by hydrogen bonding in the polymer matrix is highlighted. Water Molecules and Hydrogen Bonding Can you label the atoms partial charges and types of bonds associated with these water molecules. Water molecules have a bent structure.
Reset Help I Hydrogen Slightly negative charge Water molecule Oxygen Slightly positive charge Hydrogen bond Submit Request Answer W х. Hydrogen Bonding A low-energy attractive force between hydrogen and another element. A hydrogen atom attached to a relatively electronegative atom is a hydrogen bond donor.
These activities introduce hydrogen bonds as intermolecular bonds made between specific permanent dipoles. In water each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. Computational chemists in the US have modelled the strongest possible hydrogen bond for a neutral water cluster by manipulating how adjacent bonds cooperate.
Molecular dynamics simulations reveal the need to treat the distribution of the shared proton in the hydrogen bond quantum mechanically to capture the structural dynamics on femtosecond timescales. Help students explore hydrogen bonding and discover where hydrogen bonds are found using this lesson plan with activities for 1618 year olds. The sulfur atoms in hydrogen sulfide are bigger with 16 outermost electrons arranged in three orbits around its positive nucleus.
Adhesion is the attraction of molecules of one kind for molecules of a different kind and it can be quite strong for water especially with other molecules bearing positive or negative charges. The bond between hydrogen and oxygen is 105 which is slightly less than the idealized 1095 degree angle of sp3 hybridized orbitals. GET 20 OFF GRADE YEARLY SUBSCRIPTION.
Ionic bond O hydrogen bond nonpolar. The hydrogen bond is an attractive interaction between a hydrogen atom from a molecule or a molecular fragment XH in which X is more electronegative than H and an atom or a group of atoms in the same or a different molecule in which there is. The most simple water molecular H2O is a fascinating but poorly understood molecule.
The results from the study of the clusters with eight ten and twelve molecules are used to predict possible low-energy structures for various shapes of clusters with up to 22 molecules. Learning through art water molecules and hydrogen bonding. A water molecule consists of two hydrogen atoms bonded to an oxygen atom and its overall structure is bent.
This is because the oxygen atom in addition to forming bonds with the hydrogen atoms also carries two pairs of unshared electrons. Molecules 02 Post Lecture alkthrough. The nucleus of the majority of hydrogen atoms is made up entirely of protons.
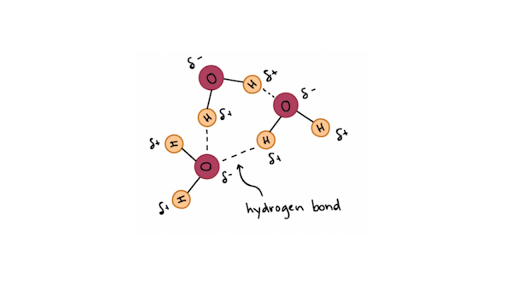
Hydrogen Bonding in Water art A e figure shows how water forms hydrogen bonds. In fact the hydrogen bonds between molecules hold each molecule further apart than would normally happen. Students work through a cognitive conflict exercise a simple experiment and then carry out research.
B Interpretation Introduction Interpretation. All of the electron pairsshared and unsharedrepel each other. 1 Water the molecule.
Water Molecules and Hydrogen Bonding Part A Drag the labels to their appropriate locations in this diagram. Ag the labels to their appropriate locations on the figure. The Hydrogen Bond and the Water Molecule offers a synthesis of what is known and currently being researched on the topic of hydrogen bonds and water molecules.
For instance adhesion enables water to climb upwards through thin glass tubes called capillary tubes placed in a beaker of water. Water contains two isotopic forms deuterium and tritium in which the atomic nuclei also contain one and two neutrons respectively. This helps in the formation of hydrogen bonding between the water molecules.
Due to the lone pair of electrons that is present on the nitrogen in the amine functional group hydrogen bonding is possible between amine molecules and also with water molecules. Learning through art water molecules and hydrogen bonding OneClass. The water molecule is made up of two hydrogen atoms that are joined together by a single chemical bond to an oxygen atom.
Water Molecules and Hydrogen Bonding 20f 10 Labels can be used once more than once or not at all Reset Help atom atom onie bond atom charge hydrogen bond polar covalent bond bond charge. In H 2 O only two of the six outer-shell electrons of oxygen are used for this purpose leaving four electrons which are organized into two non-bonding pairs. The water molecules within pure ice form hydrogen bonds with each other creating a perfect lattice structure as seen in the image below.
Learning through art water molecules and hydrogen bonding LIMITED TIME OFFER. Get the detailed answer. It plays a major role in determining the properties of water proteins and.
This makes ice less dense than liquid water which is why your ice cubes float in a glass of water. First use the labels of Group 1 to identify the atoms and charges.

Hydrogen Bonds In Water Article Khan Academy
Solved Drag The Labels To Their Appropriate Locations In Chegg Com
Reaction Of Hydrogen And Oxygen In New Compounds Water Molecule That Formed As A Result Of The Rearrangement Of Atoms Oxygen And Hydrogen Chemistry Stock Vector Image Art Alamy

Hydrogen Bond Between Water Molecules Diagram Quizlet

How Is Hydrogen Bonding Among Water Molecules Related To The Structure Of The Water Molecule Socratic Water Molecule Chemistry Education Teaching Chemistry

Enlaces Entre Moleculas De H2o Biologia Molecula Libros
Solved Can You Label The Atoms Partial Charges And Types Chegg Com
0 komentar
Posting Komentar